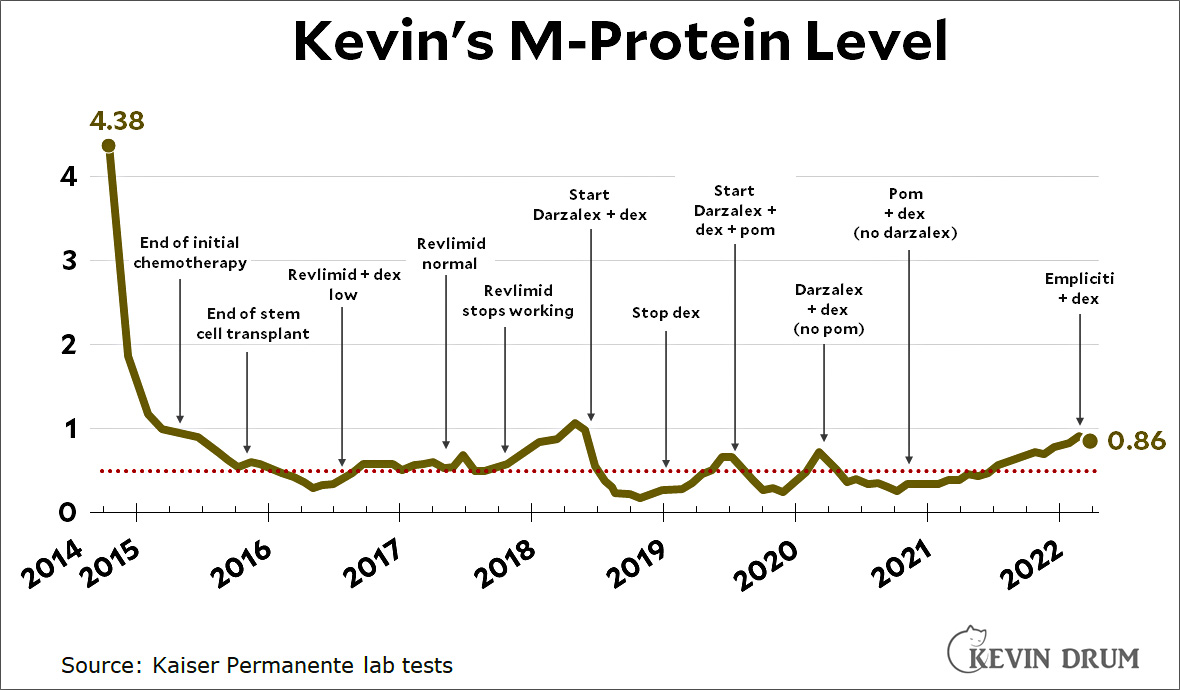
The problems with Abbott's infant formula plant in Sturgis just get mysteriouser and mysteriouser. The New York Times reports that Abbott has finally agreed with the FDA to reopen the Sturgis plant:
The agreement stems from a U.S. Department of Justice complaint and consent decree with the company and three of its executives. Those court records say the F.D.A. found a deadly bacteria, called cronobacter, in the plant in February and the company found more tranches of the bacteria later that month.
According to the complaint, the same Sturgis factory had also produced two batches of formula in the summer of 2019 and 2020 on different production equipment that tested positive for the bacteria.
Abbott staff “have been unwilling or unable to implement sustainable corrective actions to ensure the safety and quality of food manufactured for infants,” leading to the need for legal action, the documents state.
....The agreement said Abbott must hire a qualified expert to oversee a variety of improvements at the Sturgis facility.
The problems at the Sturgis plant appear to have been more severe than we've been led to believe:
Problems at the Abbott Sturgis plant surfaced in September during the F.D.A.’s first routine inspection there since the Covid-19 pandemic began. Inspectors discovered standing water inside the plant and personnel working directly with formula without proper hand hygiene, according to agency documents.
The following month, a whistleblower who worked at the plant filed a complaint under the Food Safety Modernization Act claiming that plant leaders celebrated concealing information from the F.D.A. and omitted key information from official documents.
The F.D.A. returned to the plant on Jan. 31 and discovered persistent problems, including the presence of cronobacter bacteria near production lines, according to agency records.
Obviously there's still information that none of us know. But tentatively, this sure sounds like an Abbott problem, not an FDA problem. Instead of stonewalling, Abbott could have fixed its problems in September—or, for that matter, in 2019 or 2020. But they didn't. Beyond that, they could have agreed to do what the FDA wanted back in February when they were first shut down. But it sounds like instead they argued and argued and argued, finally caving in only when the whole thing started getting lots of media attention.
It may be a while before we get the real story about Abbott's Sturgis factory, but for now all I see is the FDA trying to do its job and Abbott trying to take advantage of the FDA's limited resources in order to avoid having to do anything. Nor does it look like the FDA was being all nitpicky and unreasonable here. Abbott's plant in Columbus appears to be doing fine, after all.
Beyond this, maybe the FDA should deploy the threat of prison time for more than just three QA folks at Sturgis. Perhaps they should set their sights a little higher on the executive food chain?